When it comes to natural gas, you must not be unfamiliar with it, and nowadays no household can cook without it.The main component of natural gas is Methane, which is one of the simplest Hydrocarbon Compounds. Accelerating the development and utilization of methane is the key to realize the green and sustainable development of energy and chemical industry. In addition to its direct use as a fuel, methane can also be used as a C1 resource, that is, a molecule that contains a carbon atom and can continue to be converted to prepare high-value-added chemicals, such as methanol, formic acid and so on. Methane can be burned in oxygen to form water and carbon dioxide. Without combustion, is it possible to activate and convert the hydrocarbon bonds of methane molecules under mild conditions? The answer is yes! This is the "Holy Grail" reaction in the field of catalysis. Reactions associated with the "Holy Grail" are often extremely challenging, as they may need to be carried out under very harsh conditions, or they may need to overcome the inherent difficulties of a chemical reaction, such as the activation of highly stable compounds, low yields, and low selectivity. These challenges make it difficult to realize these reactions, but if they can be successfully achieved, they will lead to significant breakthroughs in scientific research and industrial applications.

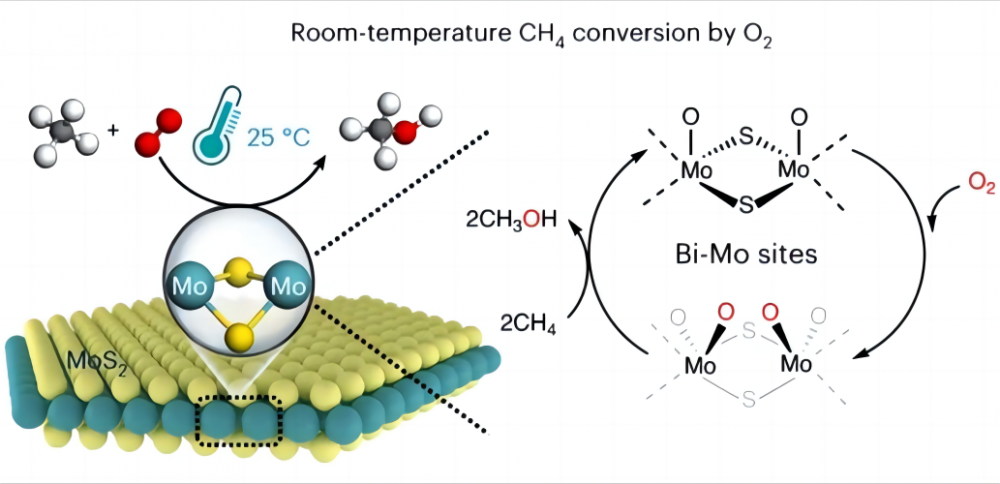
1.Challenges in the conversion of methane at low temperatures It is very difficult to convert methane directly to other useful chemicals with inexpensive oxygen at low temperatures or even room temperature, why is that? Let's look at the nature of methane and oxygen. The chemical structure of methane contains four identical carbon-hydrogen bonds (C-H) that form a highly symmetrical orthotetrahedral configuration, and each CH3-H bond of methane has a bond energy of up to 435 kJ/mol. We can think of the C-H bond of methane as a particularly strong spring. This spring is very taut and requires a lot of force to stretch. In chemistry, this "force" is the energy required to break the C-H bond. This high bonding energy makes methane's C-H bonds thermodynamically stable and very difficult to break down or react with under normal conditions. On the other hand, in chemical reactions, reactive groups are usually generated under polar interaction (polar interaction is the phenomenon that a molecule has one end positively charged and the other negatively charged), whereas the symmetric structure and nonpolar nature of the methane molecule prevents it from generating such polarity (according to the molecular configuration, a molecule with a symmetry plane has no polarity) and cannot provide reactive groups. Therefore, the activation and conversion of methane is very challenging and usually requires harsh conditions such as high temperatures (600-1100°C) or some "extremophiles" such as super-strong acids and free radicals to assist in the activation of methane. Therefore, the main difficulty in realizing the low-temperature activation of methane and oxygen lies in how to activate the C-H bond of methane, i.e., how to stretch the "spring" in the C-H bond. 2.The miracle of catalyst Scientists came up with a good solution to this problem, and chose to use a catalyst to help activate methane at low temperatures (a catalyst is a chemical that does not change before or after a reaction, but speeds up the reaction by altering the minimum amount of energy that needs to be injected for the reaction to take place). In 2023, the journal Nature Catalysis reported on the process of achieving the direct conversion of methane with oxygen to C1 oxides (methanol (CH3OH), formic acid (HCOOH), and methylene glycol (HOCH2OH)) using a specific molybdenum disulfide (MoS2) catalyst at 25°C. A methane conversion of 4.2% and almost 100% C1 oxygenates were achieved by turning methane and oxygen into valuable C1 oxygenates under ambient conditions. This MoS2 is the only catalyst reported so far that can realize the room temperature conversion of methane and oxygen. This is all due to the unique geometry and electronic structure of the Mo site on the edge of the MoS2. This Mo site has a high activation activity towards oxygen in an aqueous environment, forming the magical O=Mo=O* species. This species makes the carbon-hydrogen bond easier to break and reduces the activation energy of the C-H bond of methane, thus greatly increasing the reactivity of methane, and thus realizing the low-temperature activation of methane and oxygen. This discovery will bring more possibilities for future energy utilization and environmental protection, as well as giving us a deeper understanding of the amazing role of Catalysts And Auxiliaries.
3.Significant strategic significance of low-temperature activation of methane Realizing the direct catalytic conversion of methane and oxygen at room temperature, and converting methane in natural gas into other useful chemicals, can greatly improve the utilization rate of natural gas, reduce the waste, and better protect the environment and realize the sustainable development of energy. Secondly, as a greenhouse gas, methane is second only to carbon dioxide in its contribution to global warming. If methane can be converted into other substances, it can help us to reduce the emission of air pollutants (e.g. carbon oxides, nitrogen oxides, sulfur oxides, hydrocarbons, and Ether Compounds) and ease the pressure of global warming.
